11/30/10

The discovery came about accidentally after the scientists were looking for a way to create high-efficiency lighting similar to LED technology, but without using toxic chemicals such as phosphor powder.
The researchers infused sea urchin shaped nanoparticles of gold inside the leaves of a Bacopa Caroliniana plant. The gold reacted with the chlorophyll, causing the leaves to emit a red glow, essentially creating a bio-LED. Under a high wavelength of ultraviolet light, the gold nanoparticles were able to produce a blue-violet fluorescence to trigger a red emission in the surrounding chlorophyll.
The research is still in its infancy and there are many issues that need to be ironed out, not the least of which is the high price of gold, which makes infusing even tiny amounts quite an expensive proposition.With all these challenges these kind of streetlights are dreams with the attached questions: what happens when the tree sheds leaves?, will gold nanoparticles disrupt the ability of chlorophyll to transfer its energy to the photosynthetic pathway? and so on, but the idea is certainly intriguing and could lead to other more feasible applications.
11/30/10 by nano · 0

Cinnamon
Researchers at University of Missouri have found a method that could replace nearly all of the toxic chemicals required to make gold nanoparticles by a natural product namely cinnamon. The researchers mixed gold salts with cinnamon and stirred the mixture in water to synthesize gold nanoparticles. The new process used no electricity and utilized no toxic agents making it is a true 'green' process.
Soybean
University of Missouri research team has used soybeans as a Phytochemical Reservoir for the Production and Stabilization of Biocompatible Gold Nanoparticles.
Researchers found that by submersing gold salts in water and then adding soybeans, gold nanoparticles could be generated. The water pulls a phytochemical out of the soybean that is effective in reducing the gold to nanoparticles. A second phytochemical from the soybean, also pulled out by the water, interacts with the nanoparticles to stabilize them and keep them from fusing with the particles nearby. This process creates nanoparticles that are uniform in size in a 100-percent green process. No toxic waste was generated.
Cumin
Researchers report an unprecedented synthetic route that involves the production of well-defined spherical gold nanoparticles by simple mixing of cumin to an aqueous solution of sodium tetrachloro aurate. Production of gold nanoparticles in this cumin-mediated Green Nanotechnological process is achieved under biologically benign conditions. The gold nanoparticles generated through cumin-mediated process did not aggregate suggesting that the cocktail of phytochemicals including proteins serve as excellent coatings on nanoparticles and thus, provide robust shielding from aggregations. In addition, the phytochemical coatings on nanoparticles have rendered nontoxic features.
Gum arabic
The researchers became interested in gum arabic, a substance taken from species of the acacia tree, because it is already used to stabilize everyday foods such as yogurt, Big Macs and soda. Gum arabic has unique structural features, including a highly branched polysaccharide structure consisting of a complex mixture of potassium, calcium and magnesium salts derived from arabic acid. The scientists found that gum arabic could be used to absorb and assimilate metals and create a "coating" that makes gold nanoparticles stable and nontoxic. Gum arabic can effectively 'lock' gold nanoparticles to produce nontoxic, nanoparticulate constructs that can be used for potential applications in nanomedicine.
Black tea, turmeric, curcumin or cinnamon
In methods of the invention, an aqueous solution containing gold salts is mixed with polyphenols- or flavanoids-rich plant material. In preferred embodiment methods of making, an aqueous solution containing gold salts is provided. The aqueous solution is mixed with black tea, turmeric, curcumin or cinnamon or a similar naturally occurring polyphenols- or flavanoids-rich plant material. The gold salts react to form biocompatible gold nanoparticles that are stabilized with a coating of the polyphenols- or flavanoids-rich plant material. The black tea, turmeric, curcumin or cinnamon or similar naturally occurring polyphenols- or flavanoids-rich plant material can be a powder or can be in its root or bark form.
Pear
Korean researchers have used pear extract to obtain phytochemically-derived reducing agents for the generation and stabilization of gold nanoparticles. Pear extract is a reservoir of phytochemicals including organic acids, amino acids, peptides and proteins. In addition, the presence of saccharides in the extract provides synergistic reducing power for the rapid transformation of chloroaurate ions into gold nanoparticles. Simple mixing of pear extract (50% [v/v]) with HAuCl4 (2 mM) at 90 oC promptly initiated the appearance of a purple-red color, indicating the formation of gold nanoparticles.
Geranium extract
It has been reported that the synthesis of gold nanoparticles using geranium extract required more time to initiate and was complete in 48 h.
Emblica officinalis fruit extract
The extra cellular synthesis of highly stable Ag and Au nanoparticles has also been achieved using Emblica officinalis fruit extract.
Bacteria and fungi
Biosynthesis of silver and gold nanoparticles using bacteria, and fungi,has been reported, and the time required for completion of the reaction ranged from 24 to 120 h. Intracellular synthesis, prolonged synthesis, multiple purification steps and the maintenance of cell cultures are the drawbacks of microbial procedures.
These methods are attractive because biocompatible gold nanoparticles can be rapidly produced and stabilized under safe conditions.
by nano · 1
11/29/10
CNT based field Emission Cold Cathode
Carbon nanotubes are the best field emitters of any known material. They can be metallic as well as semi conducting, depending on the tube geometry. They are mechanically extremely stiff and resistant to bending with high electrical conductivity and sharp tip. If the tip is placed close to another electrode and a voltage is applied between the tube and electrode, a large electric field builds up near the tip of the tube and emits an electron beam. The magnitude of the electric field is inversely proportional to the radius of curvature of the tip. Thus the sharper the tip is, the larger the electric field. Even with only a few volts applied to an electrode a few microns away from the nanotube tip, electric fields in the range of a millions of Volts per centimeter will build up near the tip. These fields are large enough to pull a substantial number of electrons out of the tip. As "cold cathode" electron emitters, carbon nanotube films have been shown to be capable of emitting over 4 Amperes per square centimeter. Furthermore, the current is extremely stable.
Film field emitter (FFE) s is very useful in many applications like accelerator and high intensity X-ray sources for medical and security examinations.
However, carbon nanotubes can lead to the creation of new types of flat-panel displays. In contrast to traditional cathode ray tube monitor, where one electron gun creates the entire picture, using carbon nanotubes can instead create individual electron guns for each pixel, thus increasing the resolution of the monitor.
MWNT based field emitters
Multi-walled nanotubes (MWNTs)-based conducting polymer/metal-oxide/metal/MWNTs composites (polyaniline (PANI)/SnO2/Sn/MWNTs) have been synthesised. MWNTs can be made by chemical vapour deposition technique. SnO2/Sn/MWNTs can be prepared by using chemical reduction followed by calcinations. By in situ polymerisation method, surface of SnO2/Sn/MWNTs were coated with PANI. PANI/SnO2/Sn/MWNTs field emitters were fabricated over flexible graphitised carbon fabric substrate by spin coating technique. The fabricated PANI/SnO2/Sn/MWNTs field emitters exhibit excellent field emission properties with a turn on field of 1.83 V µm-1 and a field enhancement factor of 4800.
Samsung and Motorola besides others are using the above materials for display applications.
11/29/10 by nano · 0
11/28/10
 Carbon nanotubes hold great promise due to their extraordinary electrical, mechanical, optical, thermal, and chemical properties. Their current applications range from improving consumer electronics, to medicine delivery to cells, to strengthening airplane components. Carbon nanotubes come in many different forms and purities. They range from flexible, thin, few-walled or single-walled nanotubes (SWNTs) to rigid, long, thick, multi-walled nanotubes (MWNTs), with a spectrum of characteristics.
Carbon nanotubes hold great promise due to their extraordinary electrical, mechanical, optical, thermal, and chemical properties. Their current applications range from improving consumer electronics, to medicine delivery to cells, to strengthening airplane components. Carbon nanotubes come in many different forms and purities. They range from flexible, thin, few-walled or single-walled nanotubes (SWNTs) to rigid, long, thick, multi-walled nanotubes (MWNTs), with a spectrum of characteristics.
11/28/10 by nano · 0
11/27/10
 Researchers from the University of Massachusetts Lowell Nanomanufacturing Center of Excellence have created nano-heater elements and systems to overcome the limitations of macroscale heaters, The nano-heaters may be used in nanoscale manufacturing or on-board thermal actuation and for autonomous powering during operation of nanosized devices such as Micro and Nano-Electro-Mechanical Systems (MEMS & NEMS).
Researchers from the University of Massachusetts Lowell Nanomanufacturing Center of Excellence have created nano-heater elements and systems to overcome the limitations of macroscale heaters, The nano-heaters may be used in nanoscale manufacturing or on-board thermal actuation and for autonomous powering during operation of nanosized devices such as Micro and Nano-Electro-Mechanical Systems (MEMS & NEMS). 11/27/10 by nano · 1
11/26/10

Fabrication processes
Properties
11/26/10 by nano · 0
11/25/10
Carbon fibers
Carbon fibers are used in structural composites in aerospace, automotives, and sporting equipment, a strong fibre when added to polymer precursor. Here CNTs act as a nucleating agent for polymer crystallization to improves the polymer orientation. Incorporation of 1 wt % of nanotubes into polyacrylonitrile precursors can increase the tensile strength and modulus of the resulting carbon fiber by 64% and 49%, respectively. Plastics which are good electrical insulators, but they can be made to have electrical conductivity by loading them with conductive fillers, such as carbon black, Carbon nanotubes and graphite fibers. The natural tendency of Carbon nanotubes to form ropes provides inherently very long conductive pathways even at relatively low loadings.
Carbon nanotubes
Carbon nanotubes can be dispersed in polymers and epoxies to improve the compression, shear, and bending strengths, including stiffness, toughness, fatigue life, failure mechanisms and wear properties. Likewise nanotubes can be used to improve the modulus and toughness of elastomers and a wide variety of thermoset composite systems at loading levels of one percent or less. Carbon nanotubes blend well with plastics, and can impart reasonable conductivity at modest loadings. Carbon nanotubes have very high surface area (upto 1000 m2/g), good electrical conductivity, excellent chemical stability in acidic environments, linear geometry that makes their surface highly accessible to the electrolyte which are the desired intrinsic characteristics necessary for use as electrodes in batteries, capacitors (exceeding 100 Farads/gram), and fuel cells. Carbon nanotubes have the highest reversible capacity of any carbon material for use in lithium-ion batteries. Nanotubes could also have a significant impact on the biotech, pharmaceutical, and medical device industries. Nanotubes can be functionalized to target certain types of cells. Several research groups have published data demonstrating that nanotubes could be used as drugs or to deliver drugs to targeted cells. Nanotubes might also be used as miniature biosensors for drug discovery, diagnostics, detect small amounts of molecules through electrical, optical, or mechanical means to get specific information about targets for therapeutics.
11/25/10 by nano · 1
11/24/10
 Carbon nanotubes can be considered as graphitic sheets rolled into open cylindrical form with diameters in the range of few nanometers and lengths up to several micrometers. Each nanotube is a single molecule made up of a hexagonal network of covalently bonded carbon atoms.
Carbon nanotubes can be considered as graphitic sheets rolled into open cylindrical form with diameters in the range of few nanometers and lengths up to several micrometers. Each nanotube is a single molecule made up of a hexagonal network of covalently bonded carbon atoms.
Safety
11/24/10 by nano · 2
11/23/10
Basic books
1. Carbon Nanotubes and Related Structures New Materials for the Twenty-First Centuryby Peter J F Harris, (Department of Chemistry, University of Reading, UK), Published by Cambridge University Press and also published in China by Beijing World Publishing., 296pp Hardback ... ISBN: 0521554462. Price: £50-00/$80-00, Paperback ISBN: 0521005337. Price: £29.95/$39.95.
The new book by Peter Harris reviews the properties of carbon nanotubes and clarifies their promise as revolutionary new materials for the twenty-first century. Harris has written a most readable and useful book, which contains a wealth of up-to-date information for specialists and non-specialists. The book highlights the main challenges that have to be overcome if carbon-based nanotube technology is to fulfil the exciting, indeed revolutionary, promise that the already-known properties forecast.
2. Physical Properties of Carbon Nanotubes, English / Japanese, Authors: Riichiro Saito, Gene Dresslhaus, and M. S. Dresselhaus, Publisher: Imperial College Press (London) , ISBN 1-86094-093-5, Price: $35(hard Cover)/$24(soft cover) U.S. 258pages.
Physical Properties of Carbon Nanotubes is an introductory textbook for the graduate students and researchers who start to learn carbon nanotubes from many fields of science. Although progress in the field is still at an early stage, the book focuses on the basic principles behind the physical properties. Some computational source codes which generate coordinates of carbon nanotube are included in the textbook which will be useful to start the research.
3. Science and Application of Nanotubes, Edited by:David Tománek Richard Enbody, Kluwer Academic Publishers (available as of early March, 2000), ISBN 0-306-46372-5, David Tomanek at Michigan State University.
4. Carbon Nanotubes, Basic Concepts and Physical Properties, ISBN-10: 3-527-40386-8, ISBN-13: 978-3-527-40386-8 - Wiley-VCH, Berlin, 1. Edition - January 2004, 132.- Euro, 2004. IX, 215 Pages, Hardcover 126 Fig.- Monograph.
This text is an introduction to the physical concepts needed for investigating carbon nanotubes and other one-dimensional solid-state systems. Written for a wide scientific readership, each chapter consists of an instructive approach to the topic and sustainable ideas for solutions. The former is generally comprehensible for physicists and chemists, while the latter enable the reader to work towards the state of the art in that area. The book gives for the first time a combined theoretical and experimental description of topics like luminescence of carbon nanotubes, Raman scattering, or transport measurements. The theoretical concepts discussed range from the tight-binding approximation, which can be followed by pencil and paper, to first-principles simulations.
Nano sites
• www.thenanoage.comwww.pa.msu.edu/cmp/csc/nanotube.html,
Mirror Sites:
nanotube.msu.edu/,
www.pa.msu.edu/cmp/csc/nanotube.html
Nanotube Link Sites
University of Reading: Peter Harris' Nanotube Site
Enzo Menna at University of Padova: Library of nanotechnology links
11/23/10 by nano · 0
11/21/10
 Tungsten trioxide nanomaterials are of tremendous interest due to their potential use as electro chromic, gas sensing, and photo catalyst materials. The synthesis of doped and undoped tungsten oxide nanowires is done using either tungsten foils or powders as sources. Utilizing chemical vapor transport of metal oxide vapor species andoxygen flow over hot-filaments onto substrates, metal oxide nanowires can be fabricated.
Tungsten trioxide nanomaterials are of tremendous interest due to their potential use as electro chromic, gas sensing, and photo catalyst materials. The synthesis of doped and undoped tungsten oxide nanowires is done using either tungsten foils or powders as sources. Utilizing chemical vapor transport of metal oxide vapor species andoxygen flow over hot-filaments onto substrates, metal oxide nanowires can be fabricated. 11/21/10 by nano · 0
11/20/10
 Engineered nanomaterials have a positive impact in improving many sectors of economy, including consumer products, pharmaceutics, cosmetics, transportation, energy and agriculture etc., and are being increasingly produced for a wide range of applications within industry and research establishments. These are intentionally designed and created with physical properties tailored to meet the needs of specific applications. They can be end products in and of themselves, as in the case of quantum dots or pharmaceutical drugs, or they can be components later incorporated into separate end products, such as carbon black in rubber products. Either way, the physical properties of the particles are extremely important to their performance and the performance of any product into which they are ultimately incorporated.
Engineered nanomaterials have a positive impact in improving many sectors of economy, including consumer products, pharmaceutics, cosmetics, transportation, energy and agriculture etc., and are being increasingly produced for a wide range of applications within industry and research establishments. These are intentionally designed and created with physical properties tailored to meet the needs of specific applications. They can be end products in and of themselves, as in the case of quantum dots or pharmaceutical drugs, or they can be components later incorporated into separate end products, such as carbon black in rubber products. Either way, the physical properties of the particles are extremely important to their performance and the performance of any product into which they are ultimately incorporated.
Uses
11/20/10 by nano · 0

Nanorope structure
Carbon nanotubes can self-organize into "ropes," which consist of many (typically, 10-100) tubes running together along their length in contact with one another. Since the sides of the tubes are very smooth, carbon bonds of one tube can get relatively close to the next one and the electrons in one tube will influence the motion of some electrons in its neighbor to give rise to a short-range attractive van der Waals force between the tubes. This binding energy is approximately 0.5eV per nanometer of contact length and carbon bonds will be very difficult to separate. Such formed ropes can be far longer than any individual tube within them, but are virtually endless, branching off from one another, then joining others while individual tubes are typically about 100-2000 nm in length. In the nanoropes more than dozens of individual nanotubes are stacked together in a fairly regular pattern and looped up. These ropes are useful in providing very long electrically conductive pathways in nanotube films and composite materials.
This structure also lead to a cable or rope that has much better load transfer mechanism in tension, than a straight bundle would have. This is akin to the fact that twisted textile fibre or a steel wire is more stronger than a bundle of untwisted fibres.
The carbon nano-twist and carbon nano-rope has a structure having no space, that is, a structure having no hole in its central portion as seen from its longitudinal direction and is more stronger due to the fact that more than one carbon nano-fibers is twisted and intertwined.
Fabrication
Carbon nano-rope films can be fabricated on the Ni-catalysed Si substrate by microwave plasma-enhanced chemical vapor deposition employing a mixture of acetylene and hydrogen. These nano-ropes will be in the form of self-assembled, stranded of carbon nano-fibers aligned perpendicular to the substrate.
A carbon nano-fiber, particularly twisted carbon nano-fiber such as a carbon nano-coil, carbon nano-twist or carbon nano-rope is produced by means of a catalyst by CVD method using carbon-containing gas as a raw material and a catalyst comprising one or more components selected from the group consisting of Cr, Mn, Fe, Co, Ni and oxide thereof and one or more components selected from the group consisting of Cu, Al, Si, Ti, V, Nb, Mo, Hf, Ta, W and oxide thereof.
Application
A nano-rope made of a single type of specially chosen molecule will be very strong. A cable or rope can be assembled with incredible tensile strength with correctly-aligned nanotubes. A nanotube rope of one centimeter diameter can support the weight of one human being and weighs just 10 milligrams per kilometer.It is lightweight and invisible and can be used to create a series of invisible ropes in architecture.
by nano · 0
Nanorope device
by nano · 0
11/19/10
Carbon nanotubes hold great promise due to their extraordinary properties. Carbon nanotubes come in many different forms and purities. They range from flexible, thin, few-walled or single-walled nanotubes (SWNTs) to rigid, long, thick, multi-walled nanotubes (MWNTs), with a spectrum of characteristics and properties in-between. Carbon nanotubes can be one, two or more tubular fullerenes nested inside one another and due to the perfection of their sidewalls, these endotopic structures also form ropes.
There are currently at least five methods for producing carbon nanotubes: (1) Chemical Vapor Deposition (CVD), (2) arc discharge, (3) laser ablation, (4) HIPCO, and (5) surface mediated growth of vertically-aligned tubes by Plasma Enhanced Chemical Vapor Deposition (PECVD).
Properties
High Electrical Conductivity
Carbon nanotubes are the best conductors of electricity of any organic molecule ever discovered with a current carrying capacity per unit area that is 100 times greater than copper. Varying structures of carbon nanotubes can be defined by two indices, typically labeled n and m which specify uniquely the chirality by the ordered pair (n,m). Electronically, carbon nanotubes can be either semiconducting or metallic, depending on the value of (n-m).Tubes in which (n-m) is either zero or a multiple of 3, have electrons in their conduction bands at room temperature, conduct electricity very well, and are called metallic nanotubes. All other structures produce nanotubes that are true semiconductors, with a band gap typically between 0.5 and 3.5 electron-Volts. The band gap varies inversely with the diameter of the tube, and for a tube of 1 nm diameter, the band gap is about 1 eV. Their conductivity has been shown to be a function of their chirality (degree of twist), as well as their diameter.
Electron mobility
Carbon nanotube transistors can have a mobility that is 70 times higher than silicon.
High thermal conductivity
The thermal conductivity along the carbon nanotube is twice that of diamond (which was the best thermal conductor until the discovery of CNTs) and has a thermal conductivity of about 3000 Watts per meter-degree Kelvin. Thermal conductivity is very high along its axis because vibrations of the carbon atoms propagate easily down the tube.
Very high mechanical strength
The tensile strength of carbon nanotubes is 100 times greater than steel, but CNTs are less dense than aluminum. Nanotubes are the stiffest, strongest, and toughest molecule known, Young's modulus is up to 1.4 TPa and tensile strength is well above 100 GPa,
11/19/10 by nano · 0
11/18/10
There are literally hundreds of different carbon nanotube structures. These structures can be identified by assuming the carbon nanotube as a sheet of graphene wrapped into a seamless cylinder. But, there are many ways to do this and the cylinder can have a wide range of dimensions.
classification scheme
After the discovery of fullerene nanotubes, a classification scheme was devised to describe the different conformations of graphene cylinders. This classification scheme uses an ordered pair of numbers, (n,m), and is based upon the diagram of graphene.
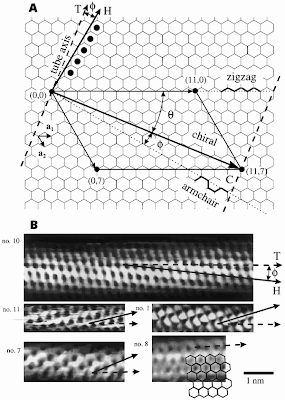 Each carbon atom in the graphene sheet is bonded to three other carbon atoms, forming a Y-shaped vertex of carbon-carbon bonds. In order to make a seamless graphene tube of a uniform diameter, the graphene sheet must be wraped in a way that permits every carbon atom in the cylinder to be bonded to three other carbon atoms where the sheet joins to itself. The number of ways this wrapping can be achieved is countable according to the numbering scheme followed. The unit vectors of the 2-dimensional graphene lattice are given as a1 and a2. Each vertex that could possibly join to the origin during a wrapping operation is labeled with an ordered pair wherein the first number of the pair is the distance (in lattice repeat units) of the vertex from the origin along a1, and the second number is the distance of the vertex from the origin along a2.
Each carbon atom in the graphene sheet is bonded to three other carbon atoms, forming a Y-shaped vertex of carbon-carbon bonds. In order to make a seamless graphene tube of a uniform diameter, the graphene sheet must be wraped in a way that permits every carbon atom in the cylinder to be bonded to three other carbon atoms where the sheet joins to itself. The number of ways this wrapping can be achieved is countable according to the numbering scheme followed. The unit vectors of the 2-dimensional graphene lattice are given as a1 and a2. Each vertex that could possibly join to the origin during a wrapping operation is labeled with an ordered pair wherein the first number of the pair is the distance (in lattice repeat units) of the vertex from the origin along a1, and the second number is the distance of the vertex from the origin along a2. 11/18/10 by nano · 0

by nano · 1
11/17/10

Nanomaterials and plants
To examine the toxicity of nanotechnology on plant functions a team of researchers from China treated cells from rice plant species Arabidopsis with carbon nanotubes to assess the viability of cells, damage to DNA, and the presence of reactive oxygen species and find the effect of nanotubes on plant cells. They found an increase in levels of the reactive oxygen species, hydrogen peroxide causes oxidative stress to cells which can cause cell death with higher dose. But cells exposed to carbon particles that were not nanotubes did not suffer any ill effects. According to the researchers "The current study has provided evidence that certain carbon nanoparticles are not 100% safe and have side effects on plants, suggesting that potential risks of nanotoxicity on plants need to be assessed,"
Another research revealed that ZnO nanoparticles at certain concentrations could adsorb onto ryegrass root surface, damage root tissues, enter root cells, and inhibit seedling growth. Inhibition of root growth varied greatly among nanoparticles and plants and partially correlated to nanoparticles concentration.
Investigation on the dissolution of ZnO nanoparticles and its contribution to the phytotoxicity revealed that in the presence of ZnO nanoparticles, ryegrass biomass significantly reduced, root tips shrank, and root epidermal and cortical cells highly vacuolated or collapsed.
Magnetic nanoparticles in small concentrations had a stimulating effect on the growth of the plantlets while the enhanced concentration of aqueous ferro fluid solution induced an inhibitory effect.
Researchers at University of Delaware, in Newark, found that pumpkin plants can take up magnetite - magnetic iron oxide - nanoparticles through their roots and that the particles are transported around the plant.
A study shows that nanoparticles are transported inside the plants and are present both in the extra cellular space and within some cells and likewise smaller Pd nanoparticles cause stress effects in leaves of a selected plant at low concentration in nutrient solution.
Certain plants take up a significant amount of magnetite nanoparticles from liquid growth medium and to accumulate them within roots and leaves particularly silver nanoparticles.
Tomato seeds exposed to CNTs germinated faster and grew into larger, heavier seedlings than other seeds.
Auburn University researchers state that tomato plants uptake the silver nanoparticles from the hydroponic solution and these silver nanoparticles led to the death of the plants. It was also found that the smaller the silver nanoparticles, the quicker the tomato plants die.
Cerium dioxide nanoparticles exposed either as aerosol or as suspension indicated that the biological barriers of plants are more resistant against nanoparticle translocation.
Scientists have also reported the first evidence that CNTs penetrate the hard outer coating of seeds, and have beneficial effects. Nanotube-exposed seeds sprouted up to two times faster than control seeds and the seedlings weighed more than twice as much as the untreated plants. Those effects may occur because nanotubes penetrate the seed coat and boost water uptake.
The phytotoxicity of nano-CeO2, nano-La2O3, nano-Gd2O3 and nano-Yb2O3 on radish, rape, tomato, lettuce, wheat, cabbage and cucumber were investigated by the Chinese researchers. They found that root growth varied greatly between different nanoparticles and plant species.
A study indicated that silver nanoparticles inhibited seed germination at lower concentrations, but showed no clear size-dependant effects, and never completely impeded germination.
Researchers at the New Jersey Institute of Technology in Newark tested and found that varying concentrations of aluminum oxide nanoparticles on hydroponically grown species cabbage, carrot, corn, cucumber and soybean resulted in a significant reduction in root growth.
It is not strange to find both positive and negative effects of nanoparticles on higher plants. It is clear that different plants have different response to the same nanoparticles. However, limited or no information is available on plant cell internalization of nanoparticles or other particles. Researches report positive or negative evidence for the toxicity of nanoparticles as it depends on their property, test organism species, and surrounding solution conditions.
Despite the scientists' observations that carbon nanotubes had toxic effects on plant cells, the use of nanotechnology in the agriculture industry still has great promise.
11/17/10 by nano · 0
11/16/10

11/16/10 by nano · 0
 Food fortification and modification
Food fortification and modificationNanotechnology will be used to transform or manufacture food from the atom up by designing and shaping molecules and atoms. Food can be fortified with nano-encapsulated nutrients, its appearance and taste can be boosted by nano-developed colors, fat and sugar content can be removed or disabled by nano-modification, and ‘mouth feel’ can be improved. Companies are designing ‘smart’ foods that will interact with consumers to ‘personalize’ food, changing color, flavor or nutrients on demand. Food ‘fortification’ means increasing the nutritional value in a processed food, for example the inclusion of ‘medically beneficial’ nano-capsules can enable chocolate chip cookies or hot chips to be marketed as health promoting or heart cleansing food item. Color, flavor, concentration and texture of the individual’s choice can be trigger released using domestic microwave oven. Nanotechnology can also change junk foods like ice cream and chocolate and modify to reduce the amount of fats and sugars that the body can absorb suitably either by replacing some of the fats and sugars with other substances, or by using nanoparticles to prevent the body from digesting or absorbing these components of the food. Vitamin and fiber-fortified fat and sugar-blocked junk food are also to be developed as health promoting and weight reducing food. Clear, tasteless drink that contains hundreds of flavors in latent nanocapsules can be developed using nanotechnology and ‘Smart’ foods can also be developed to sense when an individual is allergic to a food’s ingredients, and block the offending ingredient.
Food Packaging
Nanocomposites can be used to act as a barrier to the entry of gases such as oxygen or carbon dioxide, restrict the entry of both liquid and vapor phase chemicals and to the migration of odor and at the same time impede absorption of flavors and vitamins by the plastic packaging material itself. This barrier behavior of nanocomposites keeps products without decay and increases shelf life. Nanocomposite packaging material reduces weight of packages and thus package cost. The enhancement of shelf life and lower package cost dominate the uses of nanotechnology in food packaging.
by nano · 2
11/14/10
 Nanosensors are inherently more sensitive than any other kind of sensor, making them a future choice where lives are at stake. In addition, their small size and potentially low cost means that they can be widely deployed.
Nanosensors are inherently more sensitive than any other kind of sensor, making them a future choice where lives are at stake. In addition, their small size and potentially low cost means that they can be widely deployed.To detect very small amounts of chemical vapors carbon nanotubes, zinc oxide nanowires or palladium nanoparticles are used in nanotechnology-based sensors. These detecting elements work on the basis of changing the electrical characteristics when gas molecule strikes them. With these sensors a few gas molecules are sufficient to change the electrical properties of the sensing elements and hence the detection or monitoring is easy even with a very low concentration of chemical vapors. The goal is to make small and inexpensive sensors.
Various application of these sensors are in airport security concerns in the checking of vapors given off by explosive devices. These sensors can also be used in industrial plants to detect the release of harmful chemical vapors, leakage of hazardous chemical vapour, detection of specific gases and tracking of air pollution sources. Nanofabricated sensors are projected to reduce plant production costs since in most instances, they will be mounted on wireless packages, which eliminate wiring and cabling costs. Health, security, and environmental concerns will be major drivers for nanosensors. Security is the second driver for sensors and actuators. In the same way that nanosensors can provide ever more information on a person’s state of health, they can also provide more data to confirm a person’s identity or indicate the provenance of an object or document.
Hydrogen sensor have been developed with a layer of closely spaced palladium nanoparticles that are formed by a beading action. This works on the principle that when hydrogen is absorbed the palladium nanoparticles swell, causing short circuit between nanoparticles to lower the resistance of the palladium layer.
Sensors are made using a layer of gold nanoparticles on a polymer film for detecting volatile organic compounds (VOCs). This works on the principle that when the polymer swells in presence of VOCs, the spacing between the gold nanoparticles changes and the resistance of the gold layer changes.
Advancements in nanosensors have resulted in the invention of new-generation equipment as well as alternative technologies for detecting anthrax and other dangerous gases. Sensors using nanoporous silicon detection elements could detect chemical gas leaks or release of a toxin.
Nanotechnology Companies and their products
Company and its products
Nanomix :Carbon nanotube based sensors for detecting low levels of industrial gas. Carbon nanotube based sensors for monitoring carbon dioxide and nitric oxide levels in a patient's breath to provide a quick evaluation of their respiratory status.
Applied Nanotech: Palladium nanoparticle-based hydrogen sensor.Sensor based upon enzyme coated carbon nanotubes for analyzing chemicals in liquid samples.
Owlstone Nanotech: MEMS based sensor for detecting a wide range of gasses.
11/14/10 by nano · 0
by nano · 0
Nanotechnology is employed in food science to develop several products, from how crop is grown to how food is packaged. Companies are developing and using nanomaterials that will make a difference not only in the taste of food, but also in food safety, and the health benefits. In making lightweight bottles, cartons and packaging films, nano clay composites are being used to provide an impermeable barrier to gasses such as oxygen or carbon dioxide. Storage bins are being produced using plastics embedded with silver nanoparticles to kill bacteria from any material that was previously stored in the bins, minimizing health risks from harmful bacteria. Nanoparticles are being developed to deliver vitamins or other nutrients in food and beverages without affecting the taste or appearance. These nanoparticles encapsulate the nutrients and carry them through the stomach into the bloodstream. Researchers are using silicate nanoparticles to provide a barrier to gasses, or moisture in a plastic film used for packaging to reduce food spoiling or drying out. Zinc oxide nanoparticles are incorporated into plastic packaging to arrest UV rays providing anti bacterial protection and improve the strength and stability of the plastic film.
Nanosensors are being developed to detect bacteria and other contaminates, such as salmonella at a packaging plant. This will allow for frequent testing at a much lower cost than sending samples to a lab for analysis. This point-of-packaging testing, if conducted properly, has the potential to dramatically reduce the chance of contaminated food reaching grocery store shelves. Research is also being conducted to develop nanocapsules containing nutrients that would be released when nanosensors detect a vitamin deficiency in the human body. Basically this research could result in a super vitamin storage system in the body that delivers the nutrients needed. Interactive foods have been developed for choosing the desired flavor and color. Nanocapsules that contain flavor or color enhancers are embedded in the food; inert until a hungry consumer triggers them.
Researchers are also working on pesticides encapsulated in nanoparticles that release pesticide within an insect's stomach, minimizing the contamination of plants themselves. Another development being persued is a network of nanosensors and dispensers can be used throughout a farm field to recognize when a plant needs nutrients or water, before there is any sign that the plant is deficient. The dispensers then release fertilizer, nutrients, or water as needed, optimizing the growth of each plant in the field.
by nano · 0
11/13/10

Due to smoother edges, devices made from them have better performance characteristics. According to the researchers, the device produced from these graphene nanoribbons has a transition time three orders of magnitude shorter than those devices made from either carbon nanotubes or grapheme and key to the nanoribbon’s memory storage density is the small memory cell. The grapehene nanoribbons shrink these down to 10 nm scale.
They can be used as both static random access memories and nonvolatile flash memories cell for ultrahigh storage density applications and for digital logic gates
11/13/10 by nano · 0
by nano · 0
11/10/10
 Nanoparticles have already became an indispensable material for industries because of their unique size dependent properties such as electrical, magnetic, mechanical, optical and chemical properties, which largely differ from those of their bulk materials. Nanoparticles have an extremely high tendency of adhesion and aggregation due to different surface structures and surface interactions. Hence it is necessary to control their dispersion/aggregation phenomena of nanoparticles in order to apply them into functional materials and products.
Nanoparticles have already became an indispensable material for industries because of their unique size dependent properties such as electrical, magnetic, mechanical, optical and chemical properties, which largely differ from those of their bulk materials. Nanoparticles have an extremely high tendency of adhesion and aggregation due to different surface structures and surface interactions. Hence it is necessary to control their dispersion/aggregation phenomena of nanoparticles in order to apply them into functional materials and products.It is still a challenging issue to control the stability of suspension with highly loaded nanoparticles or sus-pensions in organic media. Surface modification of nanoparticles is one of the mostly accepted methods to improve the dispersion stability. Surface structure can be designed based on the type of nanoparticles and the liquid media in order to modify the surface by polymeric surfactants or other modifiers to generate an effective repulsive force between nanoparticles.
The adsorption of polymeric dispersant is one of the simplest surface modification techniques to improve the dispersion stability of nanoparticles in liquid media.
Surface modification of the particle surface chemically is an also an useful technique to improve the stability of nanoparticles in various liquid media. The surface modification of nanoparticles by silane coupling agents is also useful to improve the dispersion stability in organic media.
Mechanical milling method and other physical techniques such as ultrasonic dispersion can be applied to redisperse various nanoparticle into liquid media by the simultaneous processing of the surface modification cited above and the bead milling.
On preparing nanoparticles dispersible in aqueous or high-polar solvents, a polyol method is also widely accepted by many researchers.
Based on abovementioned methods, various nanoparticles including metals, oxides, sulfides, and fluorides that are redispersible into many solvents can be synthesized by in-situ surface modification techniques.
11/10/10 by nano · 0
11/9/10

Unlike typical air cleaners, nanotechnology air purifier eradicates air impurities instead of collecting them, eliminating the need to replace costly filters, and unlike commonly available ionic units, the purifier does not produce harmfully elevated ozone levels. The purifier uses nanotechnology, the same technology used to sanitize instruments and surfaces in hospitals, the air purifier being able to kill off 99% of mold spores, bacteria, pollen, and viruses. It dissolves organic vapors and gasses, such as those found in paint, insecticides, and adhesives. The integrated titanium dioxide crystals and a fluorescent bulb produce oxidizing hydroxyl radicals that destroy airborne pollutants as air is drawn into the unit.
The Filterless Nanotechnology Air Purifier is offered by Hammacher Schlemmer to improve the quality of the air in rooms and kills off the impurities in the air.
11/9/10 by nano · 3
Nanotechnology Victoria (NanoVic), a company committed to the development and promotion of nanoscience in Victoria engaged Kennovations to generate concepts for fun, educational and safe objects which both utilise and promote the practical application of nanotechnology.
NanoVic decided on two concepts as their ‘mascots - ‘Eddie the Echidna’ and ‘NanoMan’. ‘Eddie the Echidna’ features quills of NiTinol Shape Memory Alloy which react to specific environmental temperature changes. In general shape memory alloy (SMA, smart metal, memory metal, memory alloy, muscle wire, smart alloy) is an alloy that "remembers" its original, cold-forged shape: returning the pre-deformed shape by heating. This material is a lightweight, solid-state alternative to conventional actuators such as hydraulic, pneumatic, and motor-based systems. Shape memory alloys have applications in industries including medical and aerospace.Nickel Titanium (also known as Nitinol) is the unique class of materials known as shape memory alloys. A thermoelastic martensitic phase transformation in the material is responsible for its extraordinary properties.
But here the incorporation of shape memory alloy acts depending on the temperature .Below 15°C the 'Eddie the Echidna' quills can be laid flat or messed about in any position, but with the application of heat over 15°C will instantly spring back into the spiked position. ‘NanoMan’ is a super-hero polyurethane key ring featuring a battery powered UV LED backpack and chest plate impregnated with UV reactive nano-polymers.
(Source:www.hotfrog.com.au/Companies/Kennovations-Ind.)
Nanoscale Physics Research Laboratory, The University of Birmingham has developed a "Nanoman". The "Nanoman" was created by focussed electron beam deposition on the tip of an STM - an illustration of 3D nanostructure fabrication with a precision of 10nm.
by nano · 0
11/8/10
 Types of nano clay
Types of nano claySigma-Aldrich produces and markets the following : Halloysite nanoclay, Nanoclay, hydrophilic bentonite, Nanoclay, surface modified containing 0.5-5 wt. % aminopropyltriethoxysilane, 15-35 wt. % octadecylamine, surface modified containing 25-30 wt. % methyl dihydroxyethyl hydrogenated tallow ammonium, surface modified containing 25-30 wt. % octadecylamine, surface modified containing 25-30 wt. % trimethyl stearyl ammonium, surface modified containing 35-45 wt. % dimethyl dialkyl (C14-C18) amine.
Applications
The demand of nano clay is very high in the high polymer composite material market. Nano clay can also be applied in various kinds of resins, painting, thermal insulation material, plastic and ceramic materials to enhance functions and value. Nano clays provide excellent heat/ sound /electricity insulation and acid-proof function. nano clays offer high strength to the finished products.
Properties:
High wear -resistant , good durability
Heat stability
Excellent flame retard property
Low viscosity, excellent dispersing, great fluidity
Control gloss effectively
11/8/10 by nano · 0

Polymer grade (PG) montmorillonites are high purity aluminosilicate minerals sometimes referred to as phyllosilicates. They are intended for use as additives to hydrophilic polymers such as polyvinylalcohols, polysaccharides and polyacrylic acids. When fully dispersed in these host polymers they create a new category of composite materials called nanocomposites.
by nano · 0
by nano · 0
11/7/10
Courses
Available nanotechnology courses are:
Institutes offering the courses
Department of Physics and Astrophysics, University of Delhi (http://www.du.ac.in/) M. Tech. degree course in nanoscience and nanotechnology.
Bharathiar University, Coimbatore (http://www.b-u.ac.in/) M.Sc. degree course in nano science and technology.
Periyar Maniamma University, Vallam, Thanjavur (http://www.pmu.edu/) M. Tech. degree course in nanotechnology.
SRM University, Kattamkulathur (http://www.srmuniv.ac.in/) MS degree course in nano science.
Amrita Institute of Medical Science, Kochi (http://www.amrita.edu/), M.Tech. degree course in nano medical sciences and technology.
Kalasalingam University, Anand Nagar, Krishnankoil, Virudunagar, Tamil Nadu (http://www.kalasalingam.ac.in/) M.Tech. degree course in nanotechnology.
Birla Institute of Technology, Mesra, Ranchi, ME degree course with nanotechnology as an elective subject.
Delhi University, Banaras Hindu University and Indian Institute of Science (IISc.), Bangalore, have research facilities in nanotechnology leading to Ph.D.
General curriculum
Oppurtunities
General eligibility
M.Sc. degree in physics /chemistry (with mathematics up to at least Bachelor's level) / electronics science / material science / electronic instrumentation with at least 55 per cent marks in aggregate
Bachelor's degree in electrical/ mechanical/ electronics and communication/ computer engineering / instrumentation / computer science with not less than 60 per cent marks
Degree in electrical, mechanical, electronics and communication or computer engineering or M.Sc. degree course in biotechnology, computer science, physics, chemistry, electronic science, material science, electronic instrumentation
B.Tech. or B.Sc. degree in physics, chemistry, biotechnology and electronics (http://www.amity.edu/).
Source: The Hindu, Education Plus Chennai, dt Nov 8, 2010
11/7/10 by nano · 1
 The largest single usage of organoclays over recent years has been in the manufacture of polymer-clay nanocomposites. These organic–inorganic hybrid materials show superior mechanical, thermal and gas-barrier properties and water treatment. Potential benefits include increased mechanical strength, decreased gas permeability, superior flame-resistance, and even enhanced transparency when dispersed nanoclay plates suppress polymer crystallization. Organoclay is used in polymer nanocomposites and in the ink formulation to adjust the consistency of printing inks. It is used to thicken the lubricating oils to produce especially high temperature resistant lubricating greases with good working stability and water resistance. In cosmetics, organoclays are used to enhance good colour retention and coverage for nail lacquers, lipsticks and eye shadows. Nanoclays are also used as rheological modifiers in paints, inks, grease and cosmetics. Nanoclays have great potential as compared to polymer and carbon nanotubes for drug delivery applications. The organoclay treatment of waste water yields superior clarity and oil and grease removal when compared to other treatments. It also removes humic acid which is one of the common contaminant in potable water. Organoclays operate via partitioning phenomena and have a synergistic effect with activated carbon and other unit processes such as reverse osmosis. Nanoclays are known to enhance properties of many polymers giving them better clarity, stiffness, thermal stability, barrier properties to moisture, solvents, vapors, gases and flavors, reduced static cling and UV transmission in film and bottles, improved chemical, flame, scratch resistance, and dimensional stability in injection molded products. The properties of polypropylene spun bond fabrics can be enhanced by inclusion of small levels of nanoclays. Nanoclay is a wide-spectrum mycotoxin binder. Nanotechnology is now being used in the animal industry to improve the natural qualities of clay, leading the way to healthier and more productive animals. Consisting of nanosize (3.5–5.0 nm) hollow spherules, allophane is a suitable support material for enzyme immobilization. Allophane is also effective at adsorbing phenolic compounds and colour from kraft mill effluents and phosphate from water and wastewater. Organoclays are also useful in pollution control
The largest single usage of organoclays over recent years has been in the manufacture of polymer-clay nanocomposites. These organic–inorganic hybrid materials show superior mechanical, thermal and gas-barrier properties and water treatment. Potential benefits include increased mechanical strength, decreased gas permeability, superior flame-resistance, and even enhanced transparency when dispersed nanoclay plates suppress polymer crystallization. Organoclay is used in polymer nanocomposites and in the ink formulation to adjust the consistency of printing inks. It is used to thicken the lubricating oils to produce especially high temperature resistant lubricating greases with good working stability and water resistance. In cosmetics, organoclays are used to enhance good colour retention and coverage for nail lacquers, lipsticks and eye shadows. Nanoclays are also used as rheological modifiers in paints, inks, grease and cosmetics. Nanoclays have great potential as compared to polymer and carbon nanotubes for drug delivery applications. The organoclay treatment of waste water yields superior clarity and oil and grease removal when compared to other treatments. It also removes humic acid which is one of the common contaminant in potable water. Organoclays operate via partitioning phenomena and have a synergistic effect with activated carbon and other unit processes such as reverse osmosis. Nanoclays are known to enhance properties of many polymers giving them better clarity, stiffness, thermal stability, barrier properties to moisture, solvents, vapors, gases and flavors, reduced static cling and UV transmission in film and bottles, improved chemical, flame, scratch resistance, and dimensional stability in injection molded products. The properties of polypropylene spun bond fabrics can be enhanced by inclusion of small levels of nanoclays. Nanoclay is a wide-spectrum mycotoxin binder. Nanotechnology is now being used in the animal industry to improve the natural qualities of clay, leading the way to healthier and more productive animals. Consisting of nanosize (3.5–5.0 nm) hollow spherules, allophane is a suitable support material for enzyme immobilization. Allophane is also effective at adsorbing phenolic compounds and colour from kraft mill effluents and phosphate from water and wastewater. Organoclays are also useful in pollution controlCommercial brands
by nano · 0








