11/18/10
Carbon nanotube structure
Do you like this story?
There are literally hundreds of different carbon nanotube structures. These structures can be identified by assuming the carbon nanotube as a sheet of graphene wrapped into a seamless cylinder. But, there are many ways to do this and the cylinder can have a wide range of dimensions.
classification scheme
After the discovery of fullerene nanotubes, a classification scheme was devised to describe the different conformations of graphene cylinders. This classification scheme uses an ordered pair of numbers, (n,m), and is based upon the diagram of graphene.
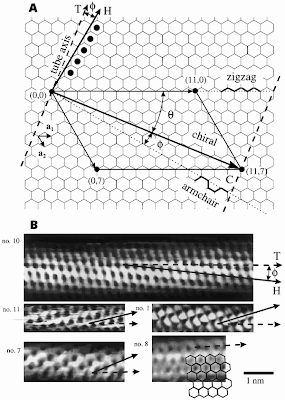 Each carbon atom in the graphene sheet is bonded to three other carbon atoms, forming a Y-shaped vertex of carbon-carbon bonds. In order to make a seamless graphene tube of a uniform diameter, the graphene sheet must be wraped in a way that permits every carbon atom in the cylinder to be bonded to three other carbon atoms where the sheet joins to itself. The number of ways this wrapping can be achieved is countable according to the numbering scheme followed. The unit vectors of the 2-dimensional graphene lattice are given as a1 and a2. Each vertex that could possibly join to the origin during a wrapping operation is labeled with an ordered pair wherein the first number of the pair is the distance (in lattice repeat units) of the vertex from the origin along a1, and the second number is the distance of the vertex from the origin along a2.
Each carbon atom in the graphene sheet is bonded to three other carbon atoms, forming a Y-shaped vertex of carbon-carbon bonds. In order to make a seamless graphene tube of a uniform diameter, the graphene sheet must be wraped in a way that permits every carbon atom in the cylinder to be bonded to three other carbon atoms where the sheet joins to itself. The number of ways this wrapping can be achieved is countable according to the numbering scheme followed. The unit vectors of the 2-dimensional graphene lattice are given as a1 and a2. Each vertex that could possibly join to the origin during a wrapping operation is labeled with an ordered pair wherein the first number of the pair is the distance (in lattice repeat units) of the vertex from the origin along a1, and the second number is the distance of the vertex from the origin along a2. For example a graphene sheet can be wrapped so that the vertex labeled (10,5) lands on top of the origin and this is classified as the (10,5) tube. In the (10,5) tube, the carbon hexagons appear to "spiral" around the tube's axis.
Conformations
The spiraling is sometimes referred to as the chirality of the tube, and the chirality is uniquely specified by the ordered pair (n,m). As do all chiral structures (such as wood screws, for example), the tubes have a "handedness". An (i,j) tube is the mirror image of a (j,i) tube, but is otherwise identical. There are two classes of nanotube conformations that are sometimes called "achiral" because their structure is completely symmetric, and the carbon hexagons do not spiral about the tube axis, but lie in lines exactly parallel to the axis. These two highly-symmetric conformations are the (n,0) (which are exactly the same as (0,n) tubes) and the (n,n) tubes. The (n, 0) tubes are often called "zig-zag" tubes since, if the tube is cut so that the open end has carbon atoms in a regular zig-zag pattern. Likewise the (n,n) tubes are sometimes referred to as armchair tubes because the carbon atoms at the cut end are in a pattern with two up, two down, and then two up again, which is reminiscent of the seat and arms of an armchair.
In production of nanotubes, there is a minimum size for the graphene sheet cylinder, which is believed to be a (4,4) tube.
Subscribe to:
Post Comments (Atom)










0 Responses to “Carbon nanotube structure”
Post a Comment